I set out this week to write a short post on how Hydrogen Peroxide works in keeping float tank water clean. The information online was so complicated that I reached out to a scientist at the Oregon Health and Science University for assistance. While much of the science is still beyond me, hopefully this post will shed some light on the chemical reaction for your own personal understanding, so you can talk with customers about how this works, as well as have a better understanding when trying to decide whether you should use H2O2, Chlorine, Bromine and or O-zone in your float tanks.
Using Hydrogen Peroxide (or H2O2) in our water is the primary way the Float Shoppe keeps our float tank water clean. Hydrogen Peroxide has historically been used to sterilize medical instruments, used for water treatment and even to bleach paper products.
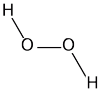
First, lets look at the structure of an H202 molecule. In it we have 2 Hydrogen atoms and 2 Oxygen Atoms.
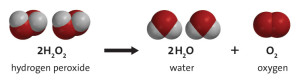
When H202 breaks down it splits creating H2O (water) and O (oxygen).
Hydrogen Peroxide decomposing into Water and Oxygen
Because water and oxygen are the elements left over from the chemical reaction, we much prefer this over Bromine or Chlorine because of the natural products left over.
The main thing I wanted to understand when researching Hydrogen Peroxide was why my skin isn't burned by laying in float tank water, but bacteria is destroyed. While the complete answer is still beyond my understanding, I did gain clarity during my conversation with my OHSU friend. The basics of it are that almost every living organism (including humans) have something called Catalase. Catalase is an enzyme (a large group of molecules that help sustain life in organisms) that facilitates the breakdown of H2O2 into water and oxygen. The decomposition of Hydrogen Peroxide creates energy that can be very harmful to organics. Catalase not only promote this reaction, but also protect an organism from damage when Hydrogen Peroxide breaks apart.. This means the reaction of H2O2 turning into H2O and O is not harmful to anything with Catalase. Most bacteria do not have this catalase. Because of this, the reaction is in fact harmful and destroys the organism.
The part of the equation that still remains a mystery to me is if harmful bacteria require harmless bacteria to be in the water to catalyze the reaction. Something we have found in our float tanks is that if the ppm (parts per million) of H2O2 reaches zero overnight, we will need to shock the tank with a large dose. During this time there is a lot of bubbling before our levels reach a normal ppm. My question is: Is the reaction occurring because of harmful bacteria or the non-harmful bacteria that have Catalase (and promote the effect of Hydrogen Peroxide breaking apart). Based on what I know, the harmless organics that have Catalase are necessary to incite the reaction that destroys the harmful bacteria.
Other Notes on H2O2
When dealing with Hydrogen Peroxide, safety should be a top priority. Most likely you will be dealing with high concentrations (we use 35%) that can burn your skin. For that reason we require our staff to use goggles and wear gloves when adding H2O2 to our water. Graham with Float Tank Solutions just published an great article on what type of gloves to use when dealing with H2O2 as well as general use in float tanks.
H2O2 has a half life, and will break down in storage. You will notice this reaction has occurred if your container is swelling. Keeping your containers in a cool or refrigerated environment will slow this process and increase the life of your H2O2.
H2O2 also has a pH of 6.7 which means it is slightly acidic, and will help balance the pH of your float tank water.